
The International Patient Decision Aid Standards (IPDAS)
Collaboration is a group of researchers, practitioners and
stakeholders from around the world that was established in 2003.
The IPDAS Collaboration is lead by professors Dawn Stacey in Canada and
Robert J Volk in the United States or America.
What is the purpose?
To enhance the quality and effectiveness of patient decision aids by establishing a shared evidence-informed framework with a set of criteria for improving their content, development, implementation, and evaluation.
These criteria are helpful to a wide variety of individuals and
organizations that use and/or develop patient decision aids. For example:
- Patients or other individuals who are making a health decision;
- Practitioners guiding patients in making health decisions;
- Developers of patient decision aids;
- Researchers or evaluators of patient decision aids;
- Policy makers or payers of patient decision aids.
Why are standards needed?
There are over 500 patient decision aids available or being developed by many
different individuals and groups around the world.
However, people have difficulty knowing whether or not a decision aid is a source of
reliable health information that can help in decision making.
What are examples of IPDAS activities?
- Maintain and revise the IPDAS criteria for improving quality and effectiveness of patient decision aids through evidence reviews.
- Provide guidance to enhance the reporting of research on patient decision aids.
- Facilitate stakeholder involvement in IPDAS. Stakeholders include patients/public, policy makers, decision aid developers, clinicians, and researchers.
- Disseminate and implement IPDAS criteria through overseeing and setting principles for:
- Use and refinement of the IPDASi instrument and its linkage to IPDAS criteria.
- Production of quality-assured IPDAS training materials for use in education events (e.g. workshops, courses, online).
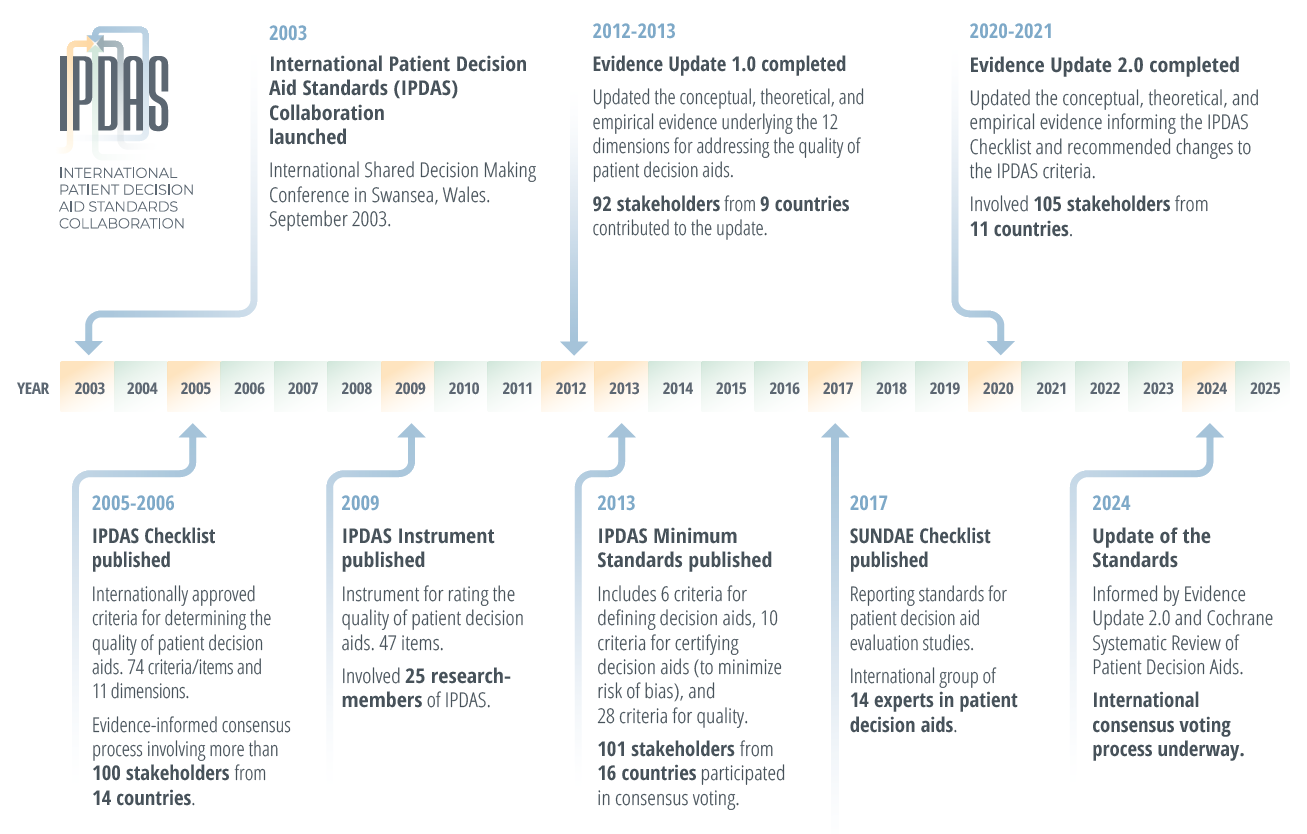
What are the results?
Publications, presentations, and supporting documents available under Resources.
- The original IPDAS Collaboration publication is:
- Glyn Elwyn, Annette O'Connor, Dawn Stacey, Robert Volk, Adrian Edwards, Angela Coulter, Richard Thomson, Alexandra Barratt, Michael Barry, Steven Bernstein, Phyllis Butow, Aileen Clarke, Vikki Entwistle, Deb Feldman-Stewart, Margaret Holmes-Rovner, Hilary Llewellyn-Thomas, Nora Moumjid, Al Mulley, Cornelia Ruland, Karen Sepucha, Alan Sykes, Tim Whelan, on behalf of the International Patient Decision Aids Standards (IPDAS) Collaboration. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. British Medical Journal. 2006 Aug 26;333(7565):417.
Would you like to join the IPDAS Collaboration?
Then join the IPDAS email list by asking a current member to introduce you by citing your interest and expertise relevant to IPDAS.
If you don't know a member, see the Who's Involved? page.
Last modified: 2024-03-13.
© 2024 IPDAS Collaboration. Email questions or comments to ipdas@ohri.ca.